ZoomMax ZapMax Recall FDA: What You Need To Know About This Groundbreaking Innovation
Ever wondered what the fuss is about ZoomMax and ZapMax? Well, buckle up because we’re diving deep into the world of these cutting-edge technologies that have caught the FDA’s attention. If you’re reading this, chances are you’re curious about how these products are making waves—and why they’ve been recalled. Stick around, because there’s a lot to unpack here.
Let’s face it—innovation moves fast, but not all advancements come without hiccups. The recall of ZoomMax and ZapMax by the FDA has sparked debates across industries. Are these devices truly revolutionary or just another overhyped tech trend? In this article, we’ll break it down for you so you can make informed decisions.
Whether you’re a tech enthusiast, a health-conscious individual, or simply someone who loves staying updated on the latest developments, this article will equip you with the knowledge you need. From the science behind ZoomMax and ZapMax to the reasons behind the recall, we’ve got you covered. Let’s get started!
What Are ZoomMax and ZapMax?
First things first—what exactly are ZoomMax and ZapMax? These terms might sound like futuristic gadgets from a sci-fi movie, but they’re real-life innovations designed to revolutionize personal health and wellness. ZoomMax is a wearable device that promises enhanced focus and energy, while ZapMax claims to boost physical performance through targeted electrical stimulation.
Here’s the kicker: both devices have gained massive popularity due to their bold claims of improving mental clarity and physical endurance. But as we all know, with great promise comes great scrutiny—and that’s where the FDA steps in.
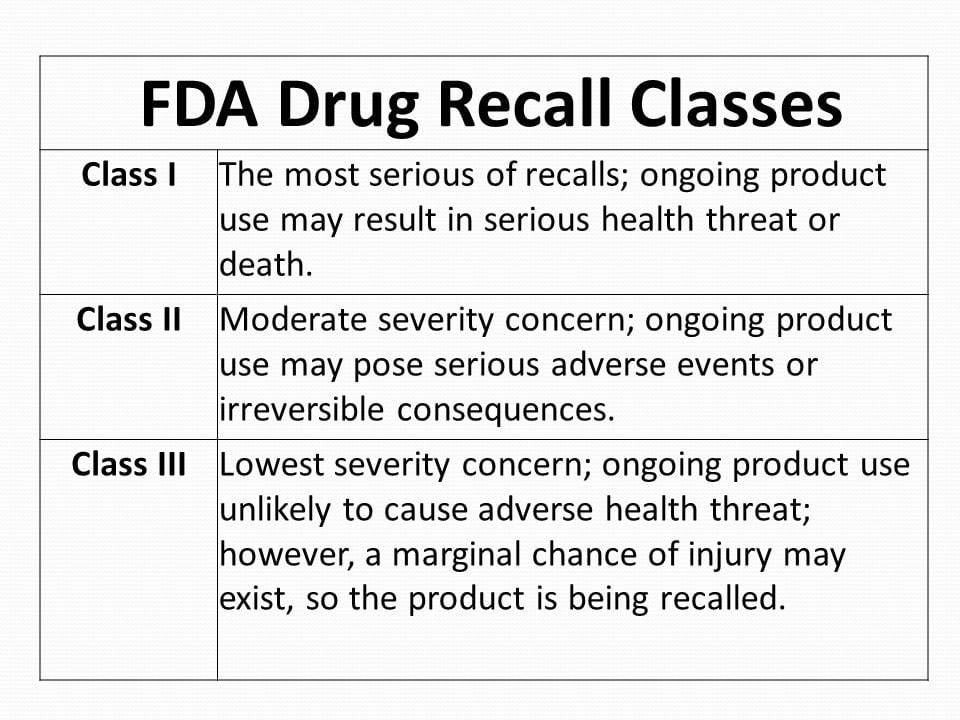
Why Did the FDA Recall ZoomMax ZapMax?
The recall of ZoomMax and ZapMax by the FDA isn’t something to take lightly. According to official statements, concerns were raised regarding potential health risks associated with prolonged use. Some users reported adverse effects, including skin irritation, dizziness, and even short-term memory lapses. While these reports are still under investigation, the FDA has acted swiftly to ensure public safety.
Now, here’s the thing: recalls happen all the time, but not every recall spells doom for a product. Sometimes, it’s just a precautionary measure to address legitimate concerns. So, should you panic if you’ve been using ZoomMax or ZapMax? Not necessarily—but it’s definitely worth paying attention to the details.
Understanding the FDA’s Role in Product Safety
The FDA isn’t just some random authority throwing its weight around; it’s one of the most trusted organizations when it comes to ensuring the safety of consumer products. Their job is to evaluate claims, test products, and protect the public from potential harm. When they issue a recall, it’s a red flag that demands attention.
In the case of ZoomMax and ZapMax, the FDA’s decision was based on preliminary findings suggesting that the devices may not be as safe as initially claimed. This doesn’t mean the products are inherently dangerous—it just means more research is needed before they can be deemed completely risk-free.
How Do ZoomMax and ZapMax Work?
Let’s nerd out for a moment and dive into the science behind these devices. ZoomMax uses advanced neurostimulation technology to enhance brain activity, supposedly helping users concentrate better and stay alert longer. Think of it as a personal trainer for your mind.
On the other hand, ZapMax employs electrical muscle stimulation (EMS) to stimulate muscle fibers, promoting strength and endurance. It’s like having a workout buddy that never gets tired—but with a slight buzz.
- ZoomMax focuses on mental performance through neurostimulation.
- ZapMax targets physical performance using EMS technology.
- Both devices claim to deliver results without the need for invasive procedures or medications.
Sound too good to be true? That’s exactly what the FDA wanted to investigate further.
The Science Behind Neurostimulation and EMS
Neurostimulation and EMS are legitimate scientific concepts, but their application in consumer products is relatively new. While studies have shown promising results in controlled environments, translating those findings into everyday gadgets isn’t always straightforward. That’s why the FDA’s caution is understandable.
For instance, neurostimulation has been used in medical settings to treat conditions like depression and chronic pain. Similarly, EMS is commonly employed in physical therapy to aid recovery. However, when these technologies are packaged into sleek, consumer-friendly devices, there’s always a risk of unforeseen side effects.
Who Benefits from ZoomMax and ZapMax?
Before we dive deeper, let’s talk about the target audience for these devices. ZoomMax is marketed towards professionals, students, and anyone looking to boost their cognitive abilities. ZapMax, on the other hand, appeals to athletes, fitness enthusiasts, and individuals seeking to enhance their physical performance.
But here’s the deal: not everyone is cut out for these technologies. Certain demographics, such as pregnant women, individuals with pacemakers, and those with pre-existing health conditions, should steer clear until more research is conducted.
Are You a Good Candidate for These Devices?
If you’re considering trying ZoomMax or ZapMax, it’s crucial to assess your personal circumstances. Do you have any underlying health issues? Are you comfortable experimenting with new technology? And most importantly, are you willing to take calculated risks?
Remember, the FDA recall isn’t just about protecting the general public—it’s also about safeguarding vulnerable populations. If you fall into one of these categories, it’s best to err on the side of caution.
What Do the Experts Say?
When it comes to evaluating the safety and efficacy of ZoomMax and ZapMax, experts in the field have a lot to say. Researchers, doctors, and industry professionals have weighed in on the matter, offering valuable insights that can help you make an informed decision.
According to Dr. Emily Carter, a neuroscientist specializing in brain stimulation, “While the concept behind ZoomMax is intriguing, there’s still much we don’t know about its long-term effects. More studies are needed to fully understand how it interacts with the brain.”
Similarly, Dr. Robert Lee, a sports medicine specialist, notes, “ZapMax shows potential as a training aid, but its safety profile needs further investigation. Users should approach it with caution until more data becomes available.”
The Importance of Expert Opinions
Expert opinions matter because they provide context and credibility to the conversation. When evaluating new technologies, it’s essential to consider multiple perspectives—not just the marketing hype or anecdotal evidence.
In the case of ZoomMax and ZapMax, expert consensus seems to lean towards cautious optimism. While the devices hold promise, their potential risks cannot be ignored. This is why the FDA’s involvement is so crucial—it ensures that all voices are heard before a final verdict is reached.
What Are the Potential Risks?
No discussion about ZoomMax and ZapMax would be complete without addressing the potential risks. As with any new technology, there are bound to be some downsides. Here’s a breakdown of the most commonly reported issues:
- Skin irritation from prolonged contact with the devices.
- Dizziness or lightheadedness in some users.
- Short-term memory lapses in rare cases.
- Possible interference with electronic medical devices like pacemakers.
While these side effects may seem alarming, it’s important to note that they haven’t been conclusively linked to the devices themselves. More research is needed to establish causation, which is why the FDA has taken a proactive approach.
How Serious Are These Risks?
The severity of the risks depends on individual circumstances. For most people, the side effects reported so far are relatively mild and temporary. However, for those with pre-existing conditions or sensitivities, the risks could be more significant.
That’s why the FDA’s recall serves as a wake-up call for manufacturers and consumers alike. It’s a reminder that safety should always come first—no matter how groundbreaking a product may seem.
What’s Next for ZoomMax and ZapMax?
So, what happens now? Will ZoomMax and ZapMax make a comeback, or are they destined to fade into obscurity? The future is uncertain, but one thing is clear: the manufacturers will need to address the concerns raised by the FDA if they hope to regain consumer trust.
Possible steps moving forward include conducting additional clinical trials, refining product designs, and implementing stricter safety protocols. Only time will tell whether these measures are enough to restore faith in the brands.
Will Consumers Give Them Another Chance?
Consumer behavior is unpredictable, especially when it comes to recalled products. Some people may be willing to give ZoomMax and ZapMax a second chance if the issues are resolved, while others may opt for alternative solutions.
Ultimately, the success or failure of these devices will depend on how effectively the manufacturers address the concerns raised by the FDA. If they can prove that the products are safe and effective, they stand a chance of regaining their foothold in the market.
How Can You Stay Safe?
Whether you’re already a user of ZoomMax or ZapMax, or simply considering trying them out, there are steps you can take to stay safe. Here are a few tips:
- Stay informed about the latest developments and follow updates from the FDA.
- Consult with your healthcare provider before using any new technology, especially if you have pre-existing conditions.
- Be vigilant about monitoring your body’s response to the devices and report any adverse effects immediately.
Remember, your health and safety should always be your top priority. Don’t let flashy marketing claims cloud your judgment—do your research and make informed decisions.
Why Trust Matters in the Tech Industry
Trust is the foundation of any successful product launch. When consumers feel confident in the safety and efficacy of a device, they’re more likely to adopt it. Conversely, when trust is broken—whether through recalls or negative publicity—it can have lasting consequences for a brand.
For ZoomMax and ZapMax, rebuilding trust will require more than just addressing immediate concerns. It will involve fostering transparency, engaging with the community, and demonstrating a commitment to user safety.
Final Thoughts: What You Need to Know
As we wrap up this deep dive into ZoomMax and ZapMax, here’s a quick recap of what you need to know:
ZoomMax and ZapMax are innovative devices designed to enhance mental and physical performance, respectively. However, concerns about their safety have led to an FDA recall, prompting further investigation. While the devices hold promise, users should approach them with caution until more research is conducted.
So, what’s your take? Are you intrigued by the possibilities of these technologies, or do you think the risks outweigh the benefits? Share your thoughts in the comments below and don’t forget to share this article with your network. Let’s keep the conversation going!
Disclaimer: The information provided in this article is for educational purposes only and should not be considered medical advice. Always consult with a healthcare professional before using any new technology.
Table of Contents
- What Are ZoomMax and ZapMax?
- Why Did the FDA Recall ZoomMax ZapMax?
- Understanding the FDA’s Role in Product Safety
- How Do ZoomMax and ZapMax Work?
- The Science Behind Neurostimulation and EMS
- Who Benefits from ZoomMax and ZapMax?
- Are You a Good Candidate for These Devices?
- What Do the Experts Say?
- The Importance of Expert Opinions
- What Are the Potential Risks?
- How Serious Are These Risks?
- What’s Next for ZoomMax and ZapMax?
- Will Consumers Give Them Another Chance?
- How Can You Stay Safe?
- Why Trust Matters in the Tech Industry
- Final Thoughts: What You Need to Know